Which of the Following Represent a Buffer System
Identify the week acid. We have a jack which is a weak acid and then we have f minus coming from K f which is its conjugal base.
A solution that is 010 mol L-1 CH3COOH and 010 mol L-1 CH3COOLi c.

. View more similar questions or ask a new question. This is the best answer based on feedback and ratings. So we do not have both weak acid with its consequent base.
What are the major chemical buffer systems of the body. The renal tubules secrete H into the tubular fluid where most of it binds to bicarbonate ammonia and phosphate buffers. Explain aH3PO3 bNaNO3 cCH3COOH and NaCH3COO dHCl and NaOH.
So this is not a buffer system. But its so weak its weaker than water. The 2nd 1 we have any and all three and a is not a um weak acid it is.
Which of the following solutions represents a good buffer system. H3PO4 NaNO3 CH3COOH and CH3COONa HCl and NaOH. A solution that is 010 mol L-1 HF and 010 mol L-1 CH3COONa b.
At the time when the acid is added to it. Match the phrases in the left column to the appropriate blanks in the sentences on the right. A buffer consisting of H2PO4- and HPO42- helps control the pH of physiological fluids.
See the answer See the answer done loading. Which of the following represent a buffer system. This problem has been solved.
The base compound that loses or accepts hydrogen ions in a buffer system. The buffer system is a system in which the solution deny for change in the ph level ie. Which of the following represent a buffer system.
Buffer system. Check all that apply. A solution that is 010 mol L-1 NaOH and 010 mol L-1 KOH a solution that is 010 mol L-1 - 25517668.
And the nitrate is also um a week base but weaker than water. Many carbonated soft drinks also use this buffer system. What is the pH of a soft drink in which the major buffer ingredients are 720 g.
C as it is a. Which of the following represent a buffer system. Consider the buffer system of hydrofluoric acid HF and its salt NaF HF aq H 2 O l H 3 O aq F - aq i The purpose of this buffer system is to.
Many carbonated soft drinks also use this buffer system. But its so weak its weaker than water. So we do not have both weak acid with its consequent base.
The respiratory system influences the acid-base balance by utilizing the A phosphate buffer system B respiratory buffer system C protein buffer system D. Which of the following represents a buffer system. So this is a buffer solution for the next one.
The 2nd 1 we have any and all three and a is not a um weak acid it is. Which of the following solutions represents a good buffer system. This problem has been solved.
It can be made either in a weak acid and salt or can be a weak base and its salt. And this would not be a buffer solution. A buffer consisting of H2PO4 and HPO42 helps control the pH of physiological fluids.
Why or why not. So this is not a buffer system. And the nitrate is also um a week base but weaker than water.
Reset Help it is a salt of a strong acid and a strong base A system with both H2C2O4 and K2C2O4 is classified as a buffer system because it contains a weak acid and its salt is not A system with both HBr and KOH classified as a buffer system because it is missing the salt of the weak acid it is a mixture of a strong acid and a strong base A system with only H2CO3 classified as a. A solution that is 010 mol L-1 HCl and 010. Which of these represents a buffer system H2CO3 and NaHCO3 KOH and KCl HCl and HF HBr and CaOH2.
A- HCl and LiCl b- NaCH3COO and H2CO3. See the answer See the answer done loading. A physiological buffer is a system namely the digestive system that stabilizes pH by controlling the bodys output of acids bases or CO2.
The next one we have a CD kassid and acid ate. This is a buffer solution. So this is not a buffer system.
Check all that apply. Can the following pairs form buffer solutions. H3PO4 H2PO4-Which if any of the following aqueous mixtures would be a buffer system.
Which of these represents a buffer system. The last one we had K c l assault and we have sodium chloride assault. See the answer See the answer done loading.
Which of the following represent a buffer system. Now when the acid is added to a buffer so the ratio is not impacted due to which it does not change sufficient for pH level. So this is not a buffer system.
A solution that is 010 mol L-1 HCl and 010 mol L-1 NH4 d. A solution that resists a change in pH when acids or bases are added. H 2 CO 3 and NaHCO 3.
So we have a weak acid with its consequent base. A The urinary and respiratory b The urinary and digestive c The bicarbonate phosphate and protein d The bicarbonate nucleic acids and protein e The bicarbonate phosphate and nitrate. You were asked to prepare this buffer from K2HPO4 and KH2PO4.
A buffer composed of 050mol acetic acid and 050mol sodium acetate is diluted to a volume of 10L. The next one we have a CD kassid and acid ate. Which of the following aqueous mixtures would be a buffer system.
A solution that is 010 mol L-1 NaOH and 010 mol L-1 KOH e. HSO4- HSO3-H2CO3 HCO3-The substance NH3 is considered.
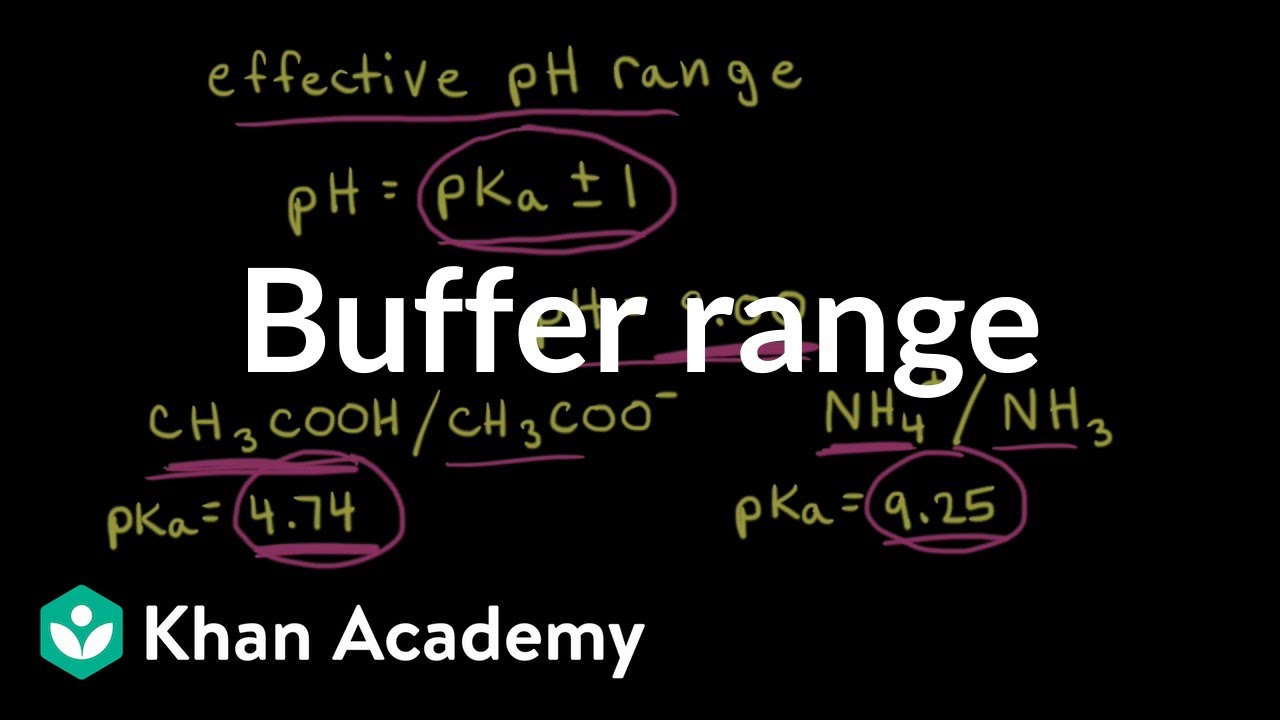
Buffer Range Video Buffers Khan Academy

Logic Gates In Details Name Graphic Symbol Algebraic Function Truth Table Electrical Discrete Mathematics Electronic Engineering Electrical Engineering
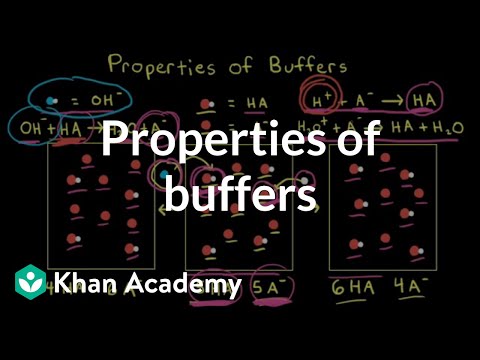
Properties Of Buffers Video Buffers Khan Academy
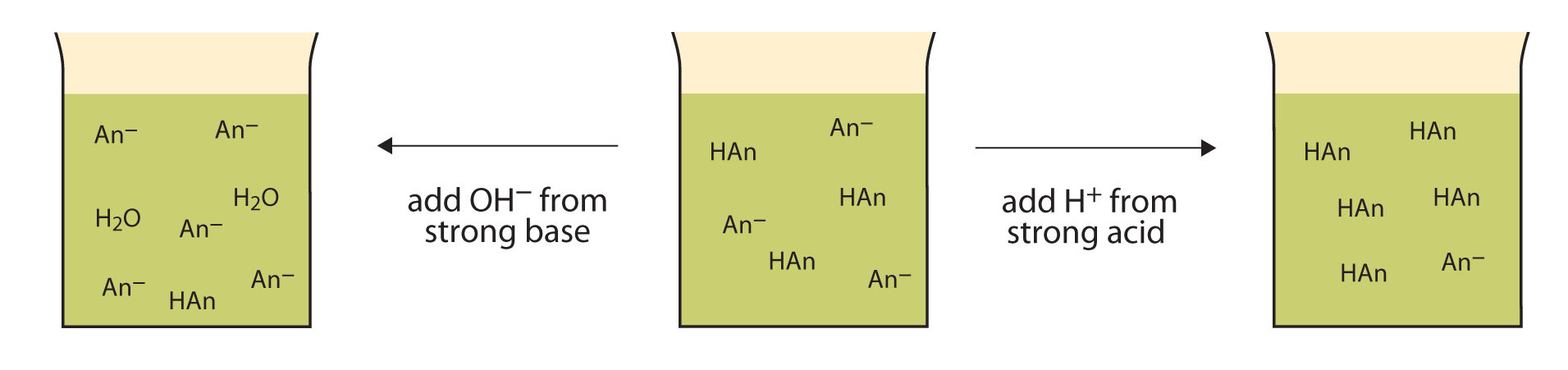
7 7 Buffers And Conjugate Acid Base Pairs Chemistry Libretexts
No comments for "Which of the Following Represent a Buffer System"
Post a Comment